Benlysta is the first new drug approved for Lupus in 50 years. It's not for everyone with Lupus, but for those who can benefit, that's awesome! Now, I know some are thinking, I don't have Lupus, why should I care about Benlysta? You don't have Lupus - why are you writing an article about Benlysta. W
[ 3 comments ] | [ read more ]
March 9, 2011 – I will remember this day, Benlysta was approved and hope was born.
I will never forget the day I was diagnosed with Lupus. That was 18 years ago, and I am now 33. For those of you doing the math, that means I have had Lupus over half my life. Right now Lupus is incurable, so I exp
[ 32 comments ] | [ read more ]

FDA approves Benlysta to treat lupus
First new lupus drug approved in 56 years
The U.S. Food and Drug Administration today approved Benlysta (belimumab) to treat patients with active, autoantibody-positive lupus (systemic lupus erythematosus) who are receiving standard therapy, includi
[ 7 comments ] | [ read more ]
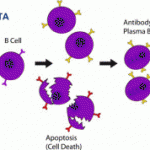
The U.S. FDA Arthritis Advisory Committee voted 13 to 2 to recommend approvalof the treatment developed by Human Genome Sciences (HGS) and GlaxoSmithKline
Tuesday, November 16, 2010
The Lupus Research Institute (LRI) and its National Coalition of state and local lupus organizations are pleased wit
[ 7 comments ] | [ read more ]

On November 16, 2010, the Food and Drug Administration (FDA) will hold a hearing to discuss the application to approve BENLYSTA® (belimumab) as a treatment to reduce disease activity in adults with active, autoantibody-positive lupus.
If approved, BENLYSTA® will be the first drug to be specif
[ 4 comments ] | [ read more ]

ROCKVILLE, Md.--(EON: Enhanced Online News)--Human Genome Sciences, Inc. (Nasdaq:HGSI) and GlaxoSmithKline PLC (GSK) today announced that the U.S. Food and Drug Administration (FDA) has granted a priority review designation to BENLYSTA® (belimumab) as a potential treatment for systemic lupus er
[ 4 comments ] | [ read more ]








